
Biomimicry and Materials Engineering: A Perspective on Re-shaping Classroom Chemistry Education
Materials ScienceReceived 21 Aug 2025 Accepted 22 Oct 2025 Published online 23 Oct 2025
ISSN: 2995-8067 | Quick Google Scholar
Next Full Text
Digital Stethoscope in the Era of Artificial Intelligence: A Comprehensive Review in the Era of Evidence-based Clinical Studies


Received 21 Aug 2025 Accepted 22 Oct 2025 Published online 23 Oct 2025
Is classroom chemistry in need of a radical change? In nature, chemistry is entangled in a complex relationship with its surroundings. From an educator’s perspective, this could be challenging as we struggle to connect the dots in the face of a teaching curriculum that is not well coordinated to this idea.
The ‘impact’ of critical teaching and learning in chemistry education is currently under considerable debate. A growing view supports the opinion that there exists a ‘disconnect’ between chemistry and its relationship with how the field functions beyond classroom knowledge []. In hindsight, a rigid curriculum separating the birth of interdisciplinary concepts in chemistry [,] from other areas [] hinders our understanding of important correlations across the breadth of chemistry and recognition of uncharted or unexplored relationships. Such areas have an important place in the path of innovative ‘discovery’, and there is a growing need to connect and accelerate the development of a modern teaching curriculum with relevance to society. This may be symptomatic of its declining interest as a subject that begins at school and demands a greater need for teachers trained in interdisciplinary teaching [].
Educators have turned the spotlight on improving chemistry education from its roots. While instilling ‘interest’ is a necessary motivational force as an educator [], building content and re-thinking strategies around challenging themes in which chemistry forms a critical part can better position teachers to facilitate this change. Teachers play a decisive role in tuning the ‘thinking process’ of students through questioning. The development of chemistry education remains a subject of much debate in designing a universally accepted curriculum representative of a conceptually evolving subject connected to an evidence-based practice [].
Historically, chemistry education has been shaped by philosophies and ideologies that often distinguish between the art of learning in the context of teaching. At the beginning of the 19th century (1801), natural philosophy was the ‘umbrella’ term used to teach sciencia (the knowledge of science) before its fragmentation into well-established specialized disciplines shortly after. While securing a strong hold in our modern-day teaching curriculum, the concept of ‘reductionism’ implies disintegrating 'knowledge' into parts from the ‘whole’ as a guiding principle to better understand complex ideas. In efforts to revive an integrated approach to science, the ‘interdisciplinary’ concept began to unfold two decades into the 20th century []. This philosophy raised ambiguities in its pedagogical significance among the sciences [-] and questioned the systematization of science itself in the late 30’s [] and the importance of experience-based learning []. The idea of the sciences being ‘divorced’ from each other spearheaded a campaign for an integrated inquiry-based curriculum []. For example, decades of teaching classical school chemistry in terms of style and content were challenged by the few [] in the United Kingdom who considered rote learning to intellectually restrict free curiosity and the inquisitive nature of the human mind []. Steps to reform an outdated curriculum in the United States were already in motion in the 1950s and 1960’s designed to replace instructional learning with better emphasis on enquiry-based learning. Pivotal to the school of thought that originated with Herbert's philosophy [] in the 19th century, a focal point centered around the lack of a unifying theme connected to several disciplines []. Although much of this happened a long time ago, it strongly impacted on shaping of modern-day thoughts, particularly in driving convergence science. Today, much of the burden in finding solutions to interdisciplinary problems falls at the doorstep of chemistry and, more specifically, nanochemistry [].
Compartmentalized teaching of ‘disciplinary’ subjects has traditionally been adopted to break down the complexities in understanding concepts in science. In more recent times, concerns have surfaced questioning the relationship between teaching and learning from a pedagogical perspective [] by contextualizing its significance to other sciences and the global surroundings [] in the curriculum. Rigidity in the traditional chemistry curriculum requires that the subdisciplinary domains be mapped to their interdisciplinary roots, allowing transdisciplinary connections to be discovered and conceptualized []. A ‘compartmentalized notion somewhat proposes the idea that taught principles are not interchangeable or extendable across disciplines, but are domain-specific with clear-cut boundaries. This conclusion is supported by the lack of synergy between the intellectual challenges faced in school chemistry and its direct applicability to deliver solutions to pressing global problems as such as energy, health, and the environment, which lie predominantly outside the curricular boundaries. In reality, problems in society are multifaceted and are often fragmented by disciplinarians into subsystems, disengaging the interconnectivity of its parts with its surroundings that define the behaviour of a system as a whole.
In the 1920s, in constrast to the views on reductionism, a new dimension to teaching and learning was being born out of the idea of ‘systems thinking’ [-] echoing Aristotle’s 17th century philosophy that the “whole is greater than the sum of its parts”. If chemistry is infact a sub-system of a number interconnected disciplines both prinicipally and fundamentally, fragmenting the ‘whole’ into its constituents parts such that the summation of the parts in their isolation falls seriously short of our understanding of the whole, is educationally damaging for the next generation of critical thinkers. Learning single processes that cannot be mapped back into the bigger system could lead to misconceptions of smaller systems, which might behave significantly differently when studied as a whole. The question that surfaces in the context of systems thinking is whether unity in the chemical principles that apply to different branches of chemistry can provide clues to uncover significant novel patterns that have gone unnoticed in their relationship to other disciplines. The current trends in shaping a green and sustainable world have to start with updating the chemistry curriculum with particular attention on ‘inter-relationships’, bringing into focus how chemistry can be better suited to solve challenging global problems by bridging chemistry outside the domain of its traditional boundaries (Figure 1a). Here, we question whether the down scaling of synthetic materials and the new properties that emerge can help drive this philosophy of integration among the sciences with nature (Figure 1b) by advancing the biomimicry of materials building a better connection with its surroundings.
Figure 1 depicts our current perception of the interdependence of chemical and biological processes, in which we perceive the art of biomimicry [] positioned in-between chemistry and biology. Indeed, the sum of the individual processes that define biological interactions and their outcomes does not adhere to a simple relationship but one that is dynamically changing in the environmental ecosystem. One example is through the process of constructivism []. This vision can be considered as a ‘bottleneck’ to changing current perceptions to explore avenues not by compartmentalization of ideas but by creating models that fit in with a ‘whole-systems’ approach. This notion stipulates that processes are interconnected in some way, which might appear random in the face of complexity, but are in fact governed by rules influencing each other from large to lower scale systems. The best-known example of this is nature itself. Understanding and exploiting the cooperative relationship between complexity and interconnectivity of an ecosystem that occurs in nature and applying it as a design tool for better solutions for existing societal problems is in itself a step-by-step challenge that is best addressed in our school curriculum. To drive the idea of interdisciplinary harmony more convincingly, we bring into focus nature as a cross-disciplinary model in which chemistry lies at the core of fundamental chemical processes. In sustaining the biodiversity of life as a prime example of an interdependent ecosystem, nature instructs, biology adapts, and chemistry provides a solution. This could be described as an extraordinary collaboration between the physical elements of the surrounding environment and chemical processes that take place in smaller sub-systems with the single aim of sustaining life. Like an integrated system composed of sub-systems, no ‘single’ system in the human body operates independently of other systems, but in fact, sub-systems affect the dynamics and outcome of other sub-systems cooperatively in unison, as visualized in Figure 2. This demonstrates interdependency between different levels of organization. An example is tumor pathology, which is affected by the microenvironment of distant organs [], exemplifying the role of cellular communication in complex systems. A systems approach in cancer biology [,] stresses the importance of embracing disciplines as diverse as engineering, mathematics, physics, and machine learning in the context of understanding tumor progression. The interactions of ‘interconnected’ networks originating from different sites on diseased tissues located remotely or at a distance are further complicated by the dimensions of space and time. This reasoning favors a ‘systems thinking’ methodology. Identifying logical behavioral patterns between problem questions that appear detached in observation, systems thinking is the gateway to link events to the broader overall picture, which is difficult to visualize in the absence of clues. Hence, within this framework, isolated problems can be viewed as sub-parts of the overall system that can be differentiated by their associated patterns. However, a meaningful appreciation of a particular problem can only be realized by understanding how each constituent part relates to the system as a whole. Perhaps, the human system in all its complexity and labyrinth of inter-dependent networks and multi-level relationships, both within and outside the biological boundary, is the most challenging system to adopt a ‘systems thinking’ approach.
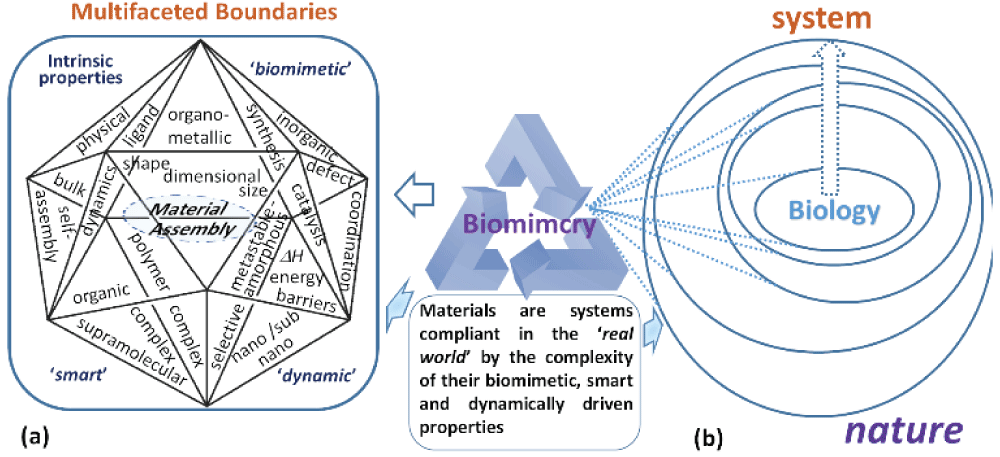 Figure 1: (a) the envisioned chemistry subsystem applicable to materials assembly showing the boundaries between the different chemical factors that may be important to material creation. (b) Material design properties imitate biological properties via biomimicry. The depth of interaction at the boundaries is a measure of its dynamic nature of the system and the extent to which biomimicry is emulated during the assembly of material(s) within a system.
Figure 1: (a) the envisioned chemistry subsystem applicable to materials assembly showing the boundaries between the different chemical factors that may be important to material creation. (b) Material design properties imitate biological properties via biomimicry. The depth of interaction at the boundaries is a measure of its dynamic nature of the system and the extent to which biomimicry is emulated during the assembly of material(s) within a system.One reason in particular that continues to resonate with students of chemistry is the ‘disconnect’ of the subject from the outside world. This was recognized by leading chemistry-led organizations such as the Royal Society of Chemistry and the American Chemical Society, through which chemical concepts were proposed to expand their scope and relationship to humanity []. Educationalists have also made effective use of analogies to teach new concepts and processes in chemistry, which proved particularly adaptive in developing the problem-solving abilities of the more inquisitive minds []. However, the concept of instructor-driven analogies works favorably only if the chosen examples identify well with their partner and are properly understood in the context of how it is taught. In the era of nanotechnology, the most innovative research themes are emerging from interdisciplinary science at boundaries where chemistry is a core element in driving a path to gain better insight into these discoveries. Spurred by a multidisciplinary approach to fuel the intellectual demands for solving very challenging but diverse problems in food security and production requiring precision and smart agricultural [] and food, environmental control and improving healthcare using nanotechnologies [-] and moving towards renewable energy [], a new school of thought is emerging. In the context of complex multidisciplinary issues we face in the current era, innovative solutions to “problems cannot be solved at the same level of awareness that created them” (Albert Einstein). Hence, opportunities for critical thinking as a criterion in taught chemistry courses must be ingrained along these lines in the very fabric of this subject to increase and implement awareness of our surroundings. In this context, the burden of expectations has descended upon ‘classroom chemistry’ in addressing and rationalizing the existing conceptual barriers that disengage the ‘modern-day’ chemistry curriculum with one that focuses on student-centered learning to consolidate emerging concepts by aiding our understanding of the chemistry of complex natural phenomena. It is more crucial now in the face of new challenges to ‘cement’ early on the new generation of learners that chemistry exists in every sphere of life and to work towards a curriculum that reflects the interrelationships, closing the knowledge gap. In their analysis of chemistry education research (CER), Cooper and Stowe conclude [], in line with current opinions in the field of CER [], that effective learning constitutes formulating thoughts in one’s mind from learning experiences and not by the transfer of knowledge from the instructor. The emphasis here is to design course materials extending the existing curriculum to ’connect’ core ideas in an organized way based on evidence around us. The conceptual nature of chemistry is challenging for the average student body []. Certainly, at the nanoscale, emerging concepts at interdisciplinary boundaries are difficult to visualize, model, and understand from the bulk scale of thought []. While the study of chemistry is now strongly embedded at scales that overlap with biology, we echo concerns about progressive understanding of how atoms and molecules are contextualized in STEM education engagement []. The suggestion that scale phenomena might be more intuitively informed by using nature as a source of inquiry into new or poorly understood concepts in chemistry. Hence, this viewpoint echoes concerns that investing in new strategies to help people learn could open doors to explore more innovative ways in the discovery path of new materials directly impacting technological development []. Accelerating our access to such knowledge will bring new challenges in introducing a revised curriculum with insightful evidence based teaching skills supporting these changes. Some misconceptions in chemistry have been addressed by using models that relate microscopic behaviour with larger scale properties of matter []. Overcoming such challenges require a better understanding of the barriers to learning and to encourage creative and investigative thought patterns crucial to STEM education awareness and advancement.
Chemistry is the cornerstone to solving a growing number of cross--disciplinary problems, and this realization evokes a major strategy in ‘re-thinking’ and re-molding problems aligned to chemistry. At the submicron to quantum scale, heterogeneity and behavioral properties of materials become especially important at interfacial boundaries where reactions are fast and hierarchical. Here, the interaction of molecular clusters becomes relevant to the dynamic behavior of bio-assemblies on a time scale that better matches such particle associations due to their diminished size, high surface area, and the manipulation of matter of many different types at many different scales. The assembly of chemical building blocks in nanomaterials chemistry emulates the process of hierarchical structural assembly in biology and demonstrates how diminishing scale (Figure 3) tends towards newly acquired structural and functional behavior while maintaining diversity. Hence, cluster chemistry at the smallest scale has gained an increasingly important role in bridging connections between disciplines []. Because the dimensional aspects of materials dominate nanoscience at an unprecedented order of scale relevant to biological processes, the chemistry of nanoscience has taken a multidisciplinary path towards convergence. While the bulk scale perception is one of separateness, in reality, the interaction of diverse sub-systems within a system is an entanglement of defined patterns that must be revised into the current teaching agenda. Whether this occurs naturally in our surroundings or artificially by synthetic means, this definition by itself demonstrates how chemistry governs the physical and chemical nature of its surroundings in a manner that begins with the complex design of molecular units and how they enable a chemical or biological process to proceed and finally integrating the ‘end product’ and its effects in the biosphere (Figure 4). However, this is not an easy task because of the existing disconnections and the intellectual bridges that need to be built or repaired to essentially convert these societal problems into chemical ones, where chemistry is at the core of the solution.
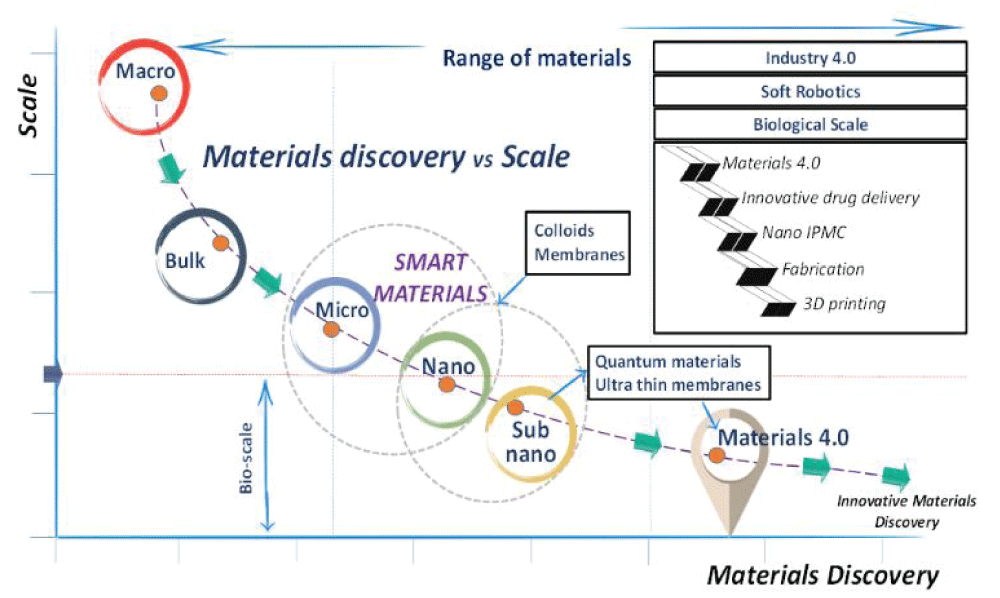 Figure 3: A visual of scale in the context of materials discovery impacting our understanding of disciplinary overlap in material behavior and the design of self-assembled complex architectures symbolic of bio-structures and their potential technological use (box inset). Reproduced with permission [].
Figure 3: A visual of scale in the context of materials discovery impacting our understanding of disciplinary overlap in material behavior and the design of self-assembled complex architectures symbolic of bio-structures and their potential technological use (box inset). Reproduced with permission [].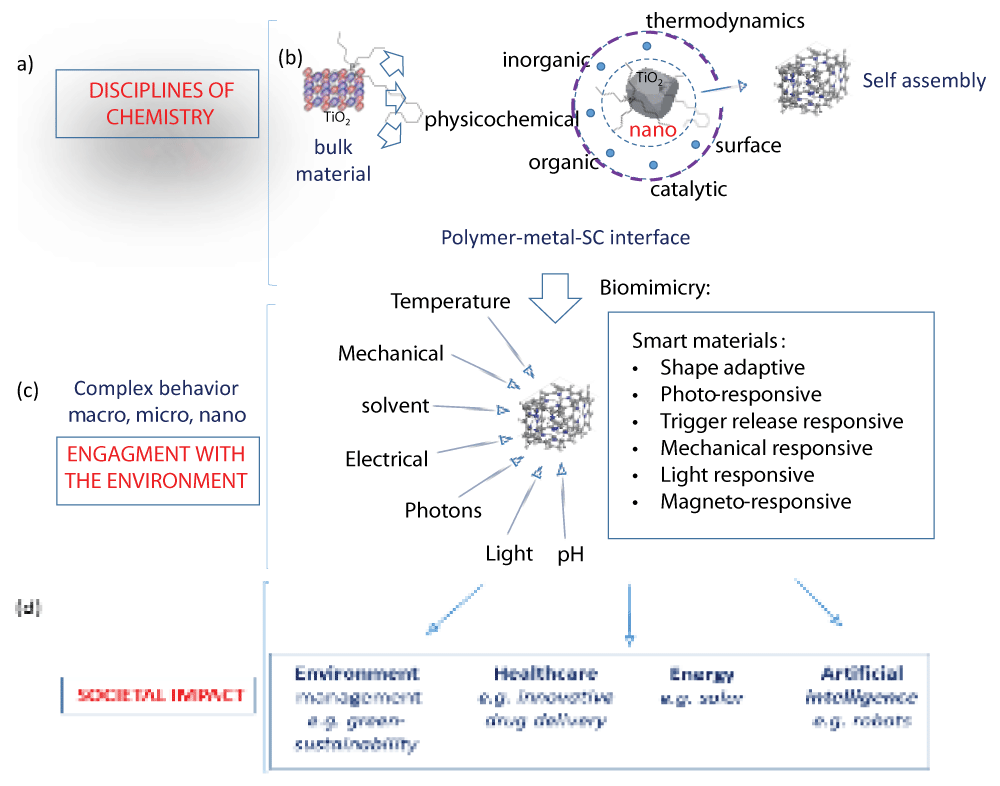 Figure 4: Systems thinking from the perspective of materials innovation in chemistry across size and scale. (a) Bulk chemistry between soft and hard materials analogous to biological environments can be used to demonstrate the biomimicry of self-assembly that results from the (b) cooperation of interdisciplinary chemistry at the metal-polymer interface. From a systems thinking view, material evolution guided by self-assembly is concurrent with the surroundings. Here, the breadth and degree of (c) smart material behavior signifies the dynamic relationship between material properties and their (d) societal impact, shaping and tuning the interplay between properties and behaviour.
Figure 4: Systems thinking from the perspective of materials innovation in chemistry across size and scale. (a) Bulk chemistry between soft and hard materials analogous to biological environments can be used to demonstrate the biomimicry of self-assembly that results from the (b) cooperation of interdisciplinary chemistry at the metal-polymer interface. From a systems thinking view, material evolution guided by self-assembly is concurrent with the surroundings. Here, the breadth and degree of (c) smart material behavior signifies the dynamic relationship between material properties and their (d) societal impact, shaping and tuning the interplay between properties and behaviour.From a pedagogical point of view, the compartmentalized approach to teaching classroom chemistry hinders the conceptual understanding of ‘whole systems and conflicts with the idea of how it might function in a multi-disciplinary sphere that connects different parts to the whole. One example is how bacteria use iron oxide from the environment and use different parts to assemble magnetic chains [] that guide the movement of the organism via magnetotaxis []. This is an example of how nature instructs, biology adapts, and chemistry executes processes that allow adaptation (Figure 5).
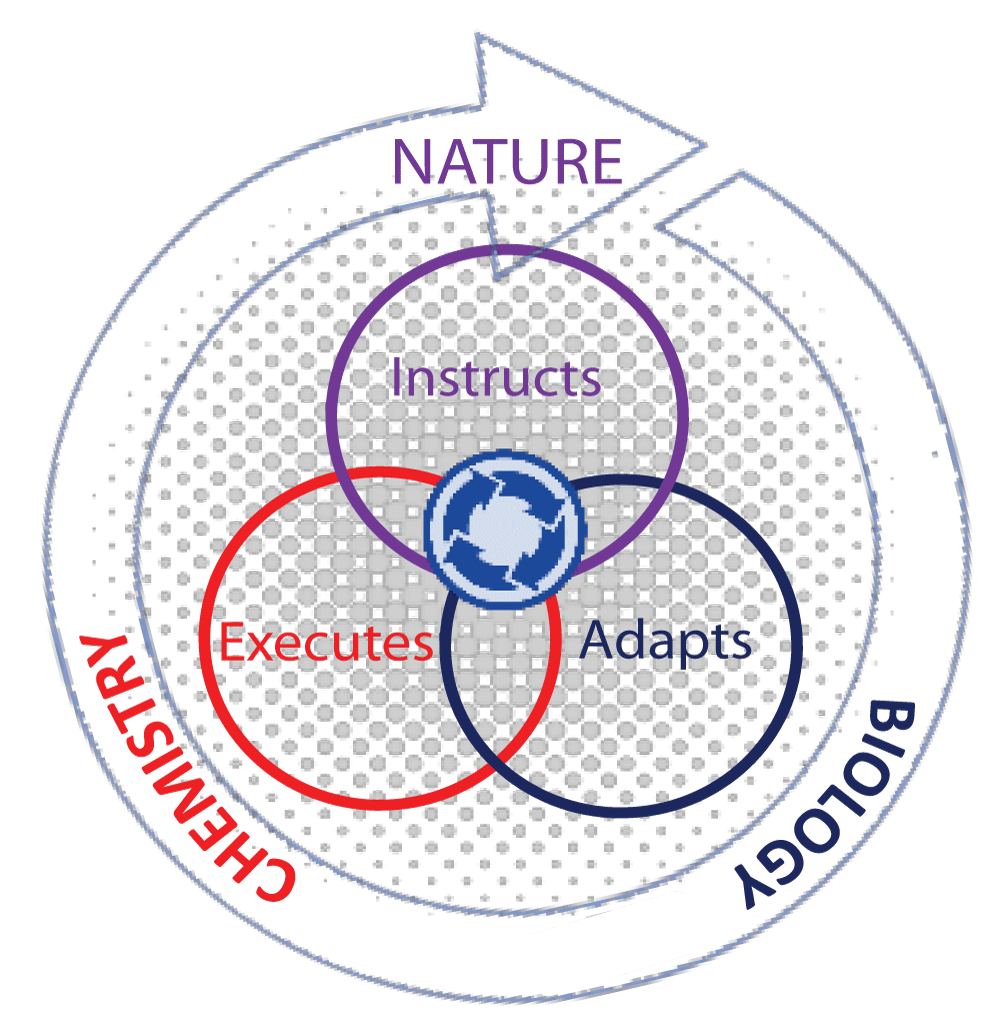 Figure 5: A model of connectivity that allows organisms to respond to nature’s instructions via genetic adaptation by acquiring minerals from their environment. Here, both biology and chemistry collaborate to solve the problem through the self-assembly of complex materials.
Figure 5: A model of connectivity that allows organisms to respond to nature’s instructions via genetic adaptation by acquiring minerals from their environment. Here, both biology and chemistry collaborate to solve the problem through the self-assembly of complex materials.This model raises several interesting but important pedagogical questions that query the transdisciplinary relationships in the context of deeper learning and how they might be more visibly taught. Can meaningful judgements of the individual components when separated explain the characteristics of the whole when combined? What is the guiding principle for teachers to help students conceptualize that the bulk chemistry of iron oxide is intrinsically altered by shape, particle size, density and surface area at the bio-chemical interface? If studying the ‘parts’ are characteristically different to the ‘whole’ when combined within a system, this introduces fundamental challenges to adapt our existing teaching methods and philosophies to allow for connections to be sought and established to explain the dynamics that surround us in the real world. Central to transdisciplinary learning to synchronize teaching strategies along these lines, it becomes necessary to:
Teaching strategies that evoke creative thinking by directing a line of thought along this theme become an important disposition to enhance the abilities of students to engage in the task of skillful thinking. For example, naturally occurring patterns are emergent [] in which the properties of the system components are not inherited by the integrated system. The properties of synthetic materials under low-dimensional control (nano and quantum scale shown in Figure 3) [] are, by this definition, emergent in their self-assembly behavior []. Because of the unpredictability of structure assembly and properties formed at the nanoscopic and sub-nanoscopic levels from bulk materials and their technological importance, how can alterations in behaviour be correlated to identifiable patterns that may be of theoretical, methodological, or visual origin to understand complex processes? How might changes at the bulk level impose changes at the supramolecular level, affecting the whole system depicted in Figure 6? One interesting example where conceptual barriers exist relates to how energy is taught in chemistry and the disconnect in its role among the disciplines. Chemistry in relation to biology is one such example []. The pedagogical argument chemistry teachers can use to drive the line of inquiry is that energy, as a scientific phenomenon, is principally the same, but is used interchangeably to drive different parts of a system to make the whole. What are the similarities in the way synthetic and non-synthetic systems use energy to assemble materials, and do they dynamically involve the same processes? A more intricate level of thought is required when asked how energy steers chemical pathways and how we might expect these pathways to converge in synthetic and non-synthetic processes. Even more interesting is how increasing surface area with diminishing scale affects material properties and performance. How does energy and its use correlate with structure? Such questions attempt to awaken the ability of students to transfer conceptual knowledge and make both visual and meaningful connections across disciplinary boundaries to what we see around us in our environments. The desired ‘knock-on’ effect for the more inquisitive minds is accessing new modes of interpretation by established connections initiated as guiding questions by teachers, during which the course of a discussion may otherwise fail to surface. An important exercise for teachers of chemistry is to reinforce this concept of ‘oneness’, and whole system integration would be better served by identifying building blocks in chemistry and re-imagining how these building blocks will behave in the context of a bio-driven system.
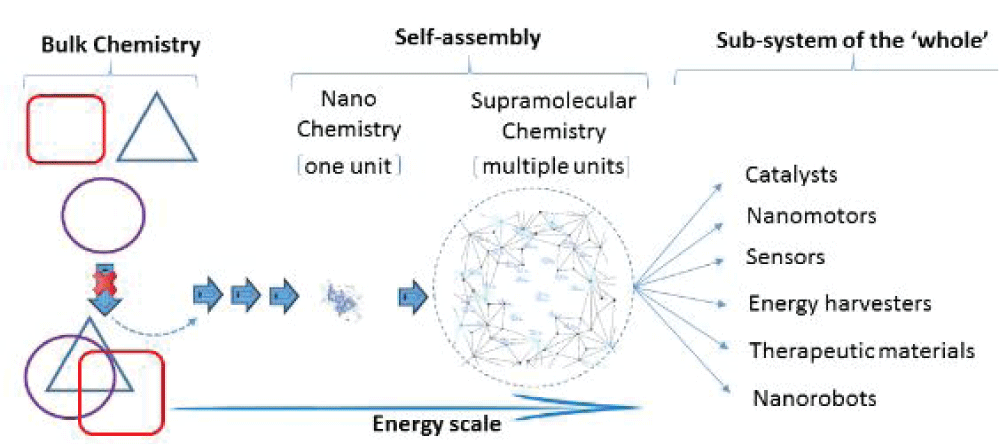 Figure 6: A schematic vision of bulk chemical components and the interdependent convergence of material behaviour of complex sub-systems at low dimensions driven through a dynamic variable network of interactions. The changes that influence the formation of the ‘sub-system’ (i.e., the low-dimensional hierarchical structure) cannot be explained by the ‘sum of the parts’ at the bulk level, particularly at the lower end of the size scale. The shape symbols representing the ‘bulk’ are no longer identifiable at the nano complex level, indicating that the parts are distinct from the whole, driven by a dynamic energy scale.
Figure 6: A schematic vision of bulk chemical components and the interdependent convergence of material behaviour of complex sub-systems at low dimensions driven through a dynamic variable network of interactions. The changes that influence the formation of the ‘sub-system’ (i.e., the low-dimensional hierarchical structure) cannot be explained by the ‘sum of the parts’ at the bulk level, particularly at the lower end of the size scale. The shape symbols representing the ‘bulk’ are no longer identifiable at the nano complex level, indicating that the parts are distinct from the whole, driven by a dynamic energy scale.This culture of thinking, reasoned by skill-based categorical questioning requiring ‘disciplinary’ jumping and compelling the student body to identify problem areas, inquire and apply concepts in a novel context, contrast and compare, examine new ideas, support interpretations with evidence-based knowledge or experimentation, leads to new approaches to problem solving. Maintaining teaching sessions that allow evolving ideas to build an understanding around concepts through strategic peer interaction and not merely led by textbook worded concepts can better serve decision-making processes.
An example of two emergent systems that drive the quantum scale synthesis of semiconductor materials is those that occur synthetically and those that are microbial-based bio-synthetic systems. An important transdisciplinary question in chemistry addresses the biomimetic significance of synthetic processes that might be aligned to produce quantum materials ambiently and in a more environmentally friendly way, as microbial organisms do. Concepts that have overlapping disciplinary significance have implications not only for addressing goals for green chemistry and sustainable materials but also for intellectual growth and technological development for tackling global problems. This is one such example that allows teachers to help students consider the vast area of system-driven interrelationships to think, conceptualize, and engineer ways to infiltrate and map hidden patterns from nature into classroom chemistry. Since biomimicry is rooted in learning and mimicking materials from nature to adopt and implement desired behavioral patterns, moving towards nature is the path of discovery, and such dynamics should also be reflected more strongly in the chemistry curriculum to support ingenuity, intuition, and independent thinking. Using ideas and prompts, a teacher could build areas of current interest as a progressive or collaborative problem-solving exercise between the students:
The emphasis should be on positioning classroom chemistry with situations more visible in nature, particularly with those problems that students and teachers alike can identify with in society, whether they concern technology, energy, health, and the environment, or other areas.
This commentary provides a perspective on improving chemistry education for teachers using a transdisciplinary approach, touching on interdisciplinary relationships between chemistry and biology and aspects of biomimicry. Chemistry is elemental to the design of materials for sustainability and growth, but this concept is not well-connected to nature and global challenges. The fragmentation of chemistry in our curriculum is now being viewed as a barrier to understanding and identifying interconnections that operate like a set of connected cog wheels, influencing and being influenced by other sub-systems. If we are to make progress in re-positioning chemistry as a science so that these connected networks can be better understood through the dynamics of biology and biomimicry as whole systems, much can be achieved. This idea must be embedded in our school chemistry courses to challenge students and to equip younger minds to conceptualize and evolve into problem solvers. As with all complex systems, a necessary step forward in introducing and implementing a ‘systems thinking’ approach must begin with identifying general patterns of behaviour that could be worked into a revised curriculum. Because nature is based on cooperativity followed by function, a model of unity among the various facets in the chemical sciences must be considered in implementing a workable and harmonious system in our curriculum.
This work acknowledges support from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (No. 2022R1A2C1006090). V.K. also thankfully acknowledge the support from the National Convergence Research of Scientific Challenges through the National Research Foundation of Korea (NRF), funded by Ministry of Science and ICT (No. 2021M3F7A1017476). S. S. and V. K. gratefully acknowledge the administrative support from the SOFT Foundry Research Institute at Seoul National University.
Olson S. Through teacher outreach programs: a workshop summary to the Chemical Sciences Roundtable. In: The high school chemistry teacher: status and outlook. Washington (DC): National Academy of Sciences; 2009;70. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26411/
Taylor EM, Mitchell R, Drennan CL. Creating an interdisciplinary introductory chemistry course without time-intensive curriculum changes. ACS Chem Biol. 2009 Dec 18;4(12):979-82. doi: 10.1021/cb9002927. PMID: 20017574; PMCID: PMC5920689.
Taniewski M. Chemistry is an interdisciplinary science and is very important for many other disciplines. Chemik. 2010;64:1–5.
Nagle B. Preparing high school students for the interdisciplinary nature of modern biology. CBE Life Sci Educ. 2013 Jun 1;12(2):144-7. doi: 10.1187/cbe.13-03-0047. PMID: 23737621; PMCID: PMC3671640.
Feszterová M. Interdisciplinary approach for the education of pre-service chemistry teachers. AIP Conf Proc. 2019;2152:030005.
Harackiewicz JM, Smith JL, Priniski SJ. Interest Matters: The Importance of Promoting Interest in Education. Policy Insights Behav Brain Sci. 2016 Oct;3(2):220-227. doi: 10.1177/2372732216655542. Epub 2016 Jun 30. PMID: 29520371; PMCID: PMC5839644.
Drinkwater MJ, Matthews KE, Seiler J. How Is Science Being Taught? Measuring Evidence-Based Teaching Practices across Undergraduate Science Departments. CBE Life Sci Educ. 2017 Spring;16(1):ar18. doi: 10.1187/cbe.15-12-0261. PMID: 28232589; PMCID: PMC5332044.
Klein JT. Interdisciplinarity: history, theory, and practice. Detroit (MI): Wayne State University Press; 1990.
Gallagher R, Appenzeller T. Beyond reductionism. Science. 1999;284:79.
Chang H. In: Arabatzis T, Renn J, Simões A, editors. Relocating the history of science: essays in honor of Kostas Gavroglu. Cham (CH): Springer International Publishing; 2015;193–209.
Anastas PT. Beyond reductionist thinking in chemistry for sustainability. Trends Chem. 2019;1:145–8.
Wrigley T. The problem of reductionism in educational theory: complexity, causality, values. Power Educ. 2019;11:145–62.
Morris CW. The unity of science movement and the United States. Synthese. 1938;3:25–9.
Dewey J. Experience and education. New York: The Macmillan Company; 1938.
Bloom BS. Ideas, problems, and methods of inquiry. In: The integration of educational experiences. Yearbook of the National Society for the Study of Education. 1958;57:84–104.
Jenkins EW. From Armstrong to Nuffield. London: John Murray; 1979.
Johnstone AH. The development of chemistry teaching. J Chem Educ. 1993;70:701.
Dunkel HB. Herbartianism comes to America: Part I. Hist Educ Q. 1969;9:202–33.
Walker DF. [Book review]. Am J Educ. 1987;95:495–8.
Meeting WCL. Chemistry in a multidisciplinary, interdisciplinary world. J Chem Educ. 2017;39:43.
Loughran J. Pedagogy: making sense of the complex relationship between teaching and learning. Curriculum Inq. 2013;43:118–41.
Fadillah A, Annisa D, Ad'hiya E, Laksmi N, Dewi C, Sadhu S. Developing students' global awareness through chemical literacy: problems and possibilities. 2018.
McGill TL, Williams LC, Mulford DR, Blakey SB, Harris RJ, Kindt JT, et al. Chemistry unbound: designing a new four-year undergraduate curriculum. J Chem Educ. 2019;96:35–46.
Fowler WC, Ting JM, Meng S, Li L, Tirrell MV. Integrating systems thinking into teaching emerging technologies. J Chem Educ. 2019;96:2805–13.
Pazicni S, Flynn AB. Systems thinking in chemistry education: theoretical challenges and opportunities. J Chem Educ. 2019;96:2752–63.
Talanquer V. Some insights into assessing chemical systems thinking. J Chem Educ. 2019;96:2918–25.
York S, Lavi R, Dori YJ, Orgill M. Applications of systems thinking in STEM education. J Chem Educ. 2019;96:2742–51.
Swiegers G, editor. Bioinspiration and biomimicry in chemistry: reverse-engineering nature. New York: John Wiley & Sons; 2012.
Bodner GM. Constructivism: a theory of knowledge. J Chem Educ. 1986;63:873.
Kamińska K, Szczylik C, Bielecka ZF, Bartnik E, Porta C, Lian F, Czarnecka AM. The role of the cell-cell interactions in cancer progression. J Cell Mol Med. 2015 Feb;19(2):283-96. doi: 10.1111/jcmm.12408. Epub 2015 Jan 19. PMID: 25598217; PMCID: PMC4407603.
Koutsogiannouli E, Papavassiliou AG, Papanikolaou NA. Complexity in cancer biology: is systems biology the answer? Cancer Med. 2013 Apr;2(2):164-77. doi: 10.1002/cam4.62. Epub 2013 Feb 17. PMID: 23634284; PMCID: PMC3639655.
Archer TC, Fertig EJ, Gosline SJ, Hafner M, Hughes SK, Joughin BA, Meyer AS, Piccolo SR, Shajahan-Haq AN. Systems Approaches to Cancer Biology. Cancer Res. 2016 Dec 1;76(23):6774-6777. doi: 10.1158/0008-5472.CAN-16-1580. Epub 2016 Nov 18. PMID: 27864348; PMCID: PMC5135591.
Linthorst JA. The image of chemistry and curriculum changes. Educ Quim. 2012;23:240–2.
Sarantopoulos P, Tsaparlis G. Analogies in chemistry teaching as a means of attainment of cognitive and affective objectives: a longitudinal study in a naturalistic setting using analogies with a strong social content. Chem Educ Res Pract. 2004;5:33–50.
Georgiou SM, Georgiou J. Precision agriculture: challenges in sensors and electronics for real-time soil and plant monitoring. 2018;1–4.
Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven challenges for nanomedicine. Nat Nanotechnol. 2008 May;3(5):242-4. doi: 10.1038/nnano.2008.114. PMID: 18654511.
Halappanavar S, Vogel U, Wallin H, Yauk CL. Promise and peril in nanomedicine: the challenges and needs for integrated systems biology approaches to define health risk. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018 Jan;10(1):e1465. doi: 10.1002/wnan.1465. Epub 2017 Mar 15. PMID: 28294555; PMCID: PMC5763403.
Hua S, Wu SY. Editorial: Advances and Challenges in Nanomedicine. Front Pharmacol. 2018 Nov 29;9:1397. doi: 10.3389/fphar.2018.01397. PMID: 30555328; PMCID: PMC6281879.
Grafton RQ, McLindin M, Hussey K, Wyrwoll P, Wichelns D, Ringler C, et al. Responding to global challenges in food, energy, environment and water: risks and options assessment for decision-making. Asia Pac Policy Stud. 2016;3:275–99.
Cooper MM, Stowe RL. Chemistry Education Research-From Personal Empiricism to Evidence, Theory, and Informed Practice. Chem Rev. 2018 Jun 27;118(12):6053-6087. doi: 10.1021/acs.chemrev.8b00020. Epub 2018 Jun 12. PMID: 29893111.
Windschitl M. Framing constructivism in practice as the negotiation of dilemmas: an analysis of the conceptual, pedagogical, cultural, and political challenges facing teachers. Rev Educ Res. 2002;72:131–75.
Taber KS. The challenge of teaching and learning chemical concepts. Cambridge (UK): The Royal Society of Chemistry; 2019.
Sebastian V, Gimenez M. Teaching nanoscience and thinking nano at the macroscale: nanocapsules of wisdom. Procedia Soc Behav Sci. 2016;228:489–95.
Bradforth SE, Miller ER, Dichtel WR, Leibovich AK, Feig AL, Martin JD, Bjorkman KS, Schultz ZD, Smith TL. University learning: Improve undergraduate science education. Nature. 2015 Jul 16;523(7560):282-4. doi: 10.1038/523282a. PMID: 26178951.
Harris HH. Chemical misconceptions—prevention, diagnosis and cure; Volume I: Theoretical background; Volume II: Classroom resources (Taber, Keith). J Chem Educ. 2003;80:491.
Castleman AW Jr, Jena P. Clusters: a bridge between disciplines. Proc Natl Acad Sci U S A. 2006 Jul 11;103(28):10552-3. doi: 10.1073/pnas.0601783103. PMID: 16835304; PMCID: PMC1502271.
Sonkaria S, Lee J, Khare V. The potential of multi-functional nanostructured components for soft robotic medical applications. In: Proceedings of the 19th International Conference on Control, Automation and Systems (ICCAS); 2019.
Sonkaria S, Fuentes G, Verma C, Narang R, Khare V, Fischer A, Faivre D. Insight into the assembly properties and functional organisation of the magnetotactic bacterial actin-like homolog, MamK. PLoS One. 2012;7(5):e34189. doi: 10.1371/journal.pone.0034189. Epub 2012 May 7. PMID: 22586444; PMCID: PMC3346761.
Lin W, Paterson GA, Zhu Q, Wang Y, Kopylova E, Li Y, Knight R, Bazylinski DA, Zhu R, Kirschvink JL, Pan Y. Origin of microbial biomineralization and magnetotaxis during the Archean. Proc Natl Acad Sci U S A. 2017 Feb 28;114(9):2171-2176. doi: 10.1073/pnas.1614654114. Epub 2017 Feb 13. PMID: 28193877; PMCID: PMC5338559.
Monat JP. Explaining natural patterns using systems thinking. Am J Syst Sci. 2018;6:1–15.
Khare V, Sonkaria S. Nanobiomimicry at quantum scales: synthetic imperfections to imitating low-dimensional biomimetic polymer confinement of TiO2 at self-assembled heterogeneous interfaces. Amsterdam: Elsevier; 2018.
Whitesides GM, Boncheva M. Beyond molecules: self-assembly of mesoscopic and macroscopic components. Proc Natl Acad Sci U S A. 2002 Apr 16;99(8):4769-74. doi: 10.1073/pnas.082065899. PMID: 11959929; PMCID: PMC122665.
Kohn KP, Underwood SM, Cooper MM. Energy Connections and Misconnections across Chemistry and Biology. CBE Life Sci Educ. 2018 Spring;17(1):ar3. doi: 10.1187/cbe.17-08-0169. PMID: 29351907; PMCID: PMC6007765.
Sonkaria S, Srinivasan SS, Khare V. Biomimicry and Materials Engineering: A Perspective on Re-shaping Classroom Chemistry Education. IgMin Res. October 23, 2025; 3(10): 387-394. IgMin ID: igmin319; DOI:10.61927/igmin319; Available at: igmin.link/p319
Anyone you share the following link with will be able to read this content:
1Soft Foundry Institute, College of Engineering, Seoul National University, Seoul, South Korea
2Department of Engineering Physics, Florida Polytechnic University, 4700 Research Way, Lakeland 33805, FL, USA
#These authors contributed equally
Address Correspondence:
Sanjiv Sonkaria, Soft Foundry Institute, College of Engineering, Seoul National University, Seoul, South Korea, Email: ssonkaria64@snu.ac.kr
How to cite this article:
Sonkaria S, Srinivasan SS, Khare V. Biomimicry and Materials Engineering: A Perspective on Re-shaping Classroom Chemistry Education. IgMin Res. October 23, 2025; 3(10): 387-394. IgMin ID: igmin319; DOI:10.61927/igmin319; Available at: igmin.link/p319
Copyright: © 2025 Sonkaria S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
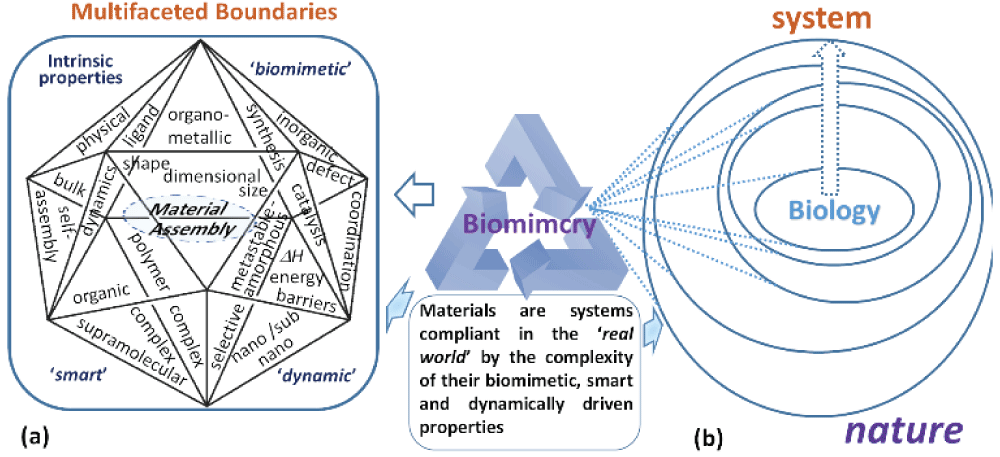 Figure 1: (a) the envisioned chemistry subsystem applicable ...
Figure 1: (a) the envisioned chemistry subsystem applicable ...
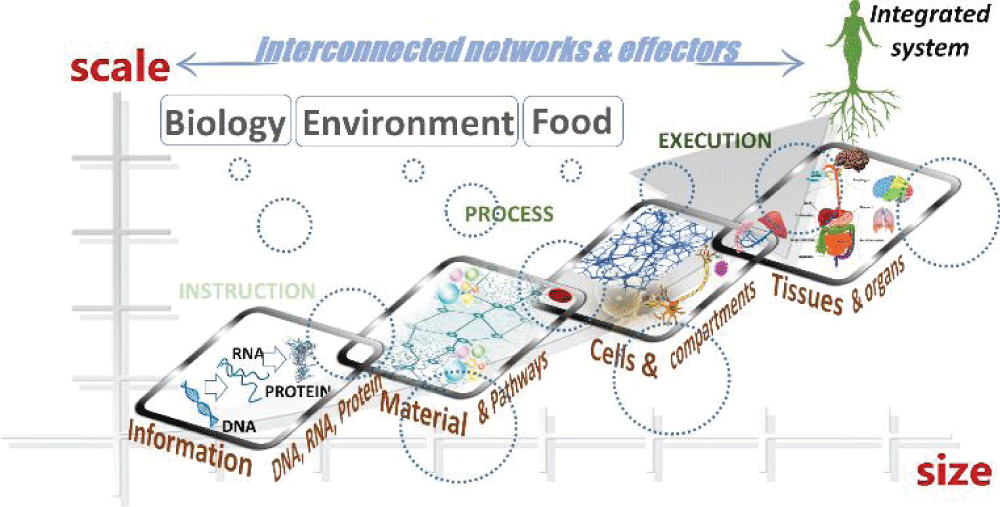 Figure 2: A visual of a ‘systems thinking’ philosophy in...
Figure 2: A visual of a ‘systems thinking’ philosophy in...
![A visual of scale in the context of materials discovery impacting our understanding of disciplinary overlap in material behavior and the design of self-assembled complex architectures symbolic of bio-structures and their potential technological use (box inset). Reproduced with permission [47].](https://www.igminresearch.es/articles/figures/igmin319/igmin319.g003.png) Figure 3: A visual of scale in the context of materials disc...
Figure 3: A visual of scale in the context of materials disc...
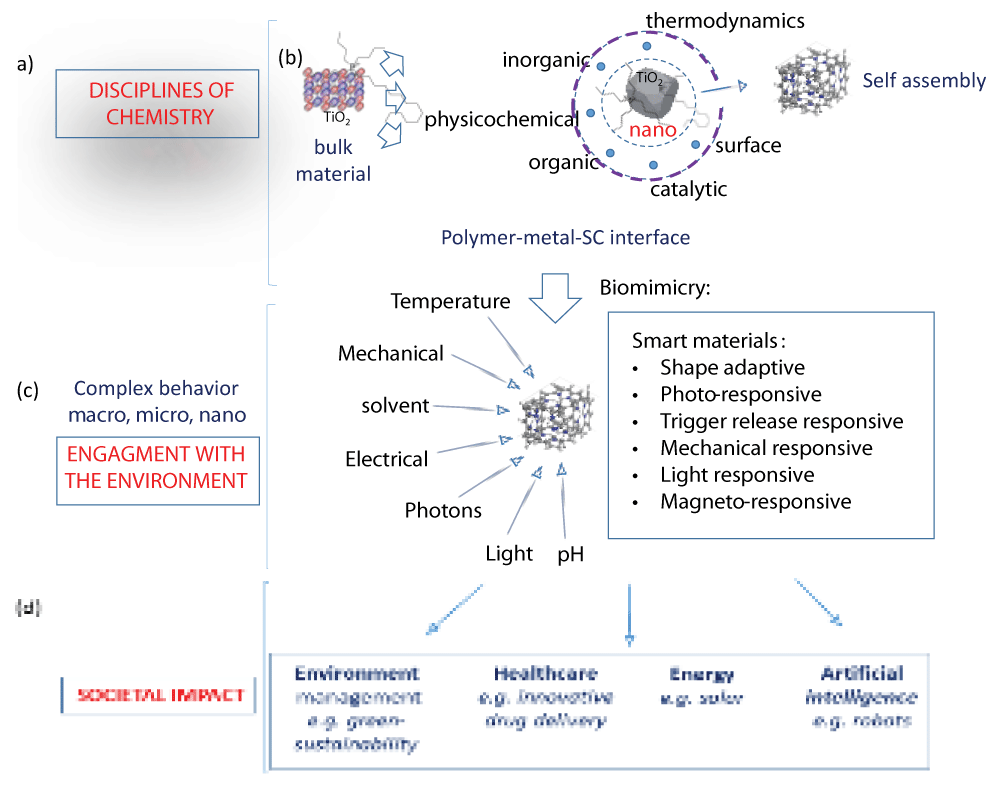 Figure 4: Systems thinking from the perspective of materials...
Figure 4: Systems thinking from the perspective of materials...
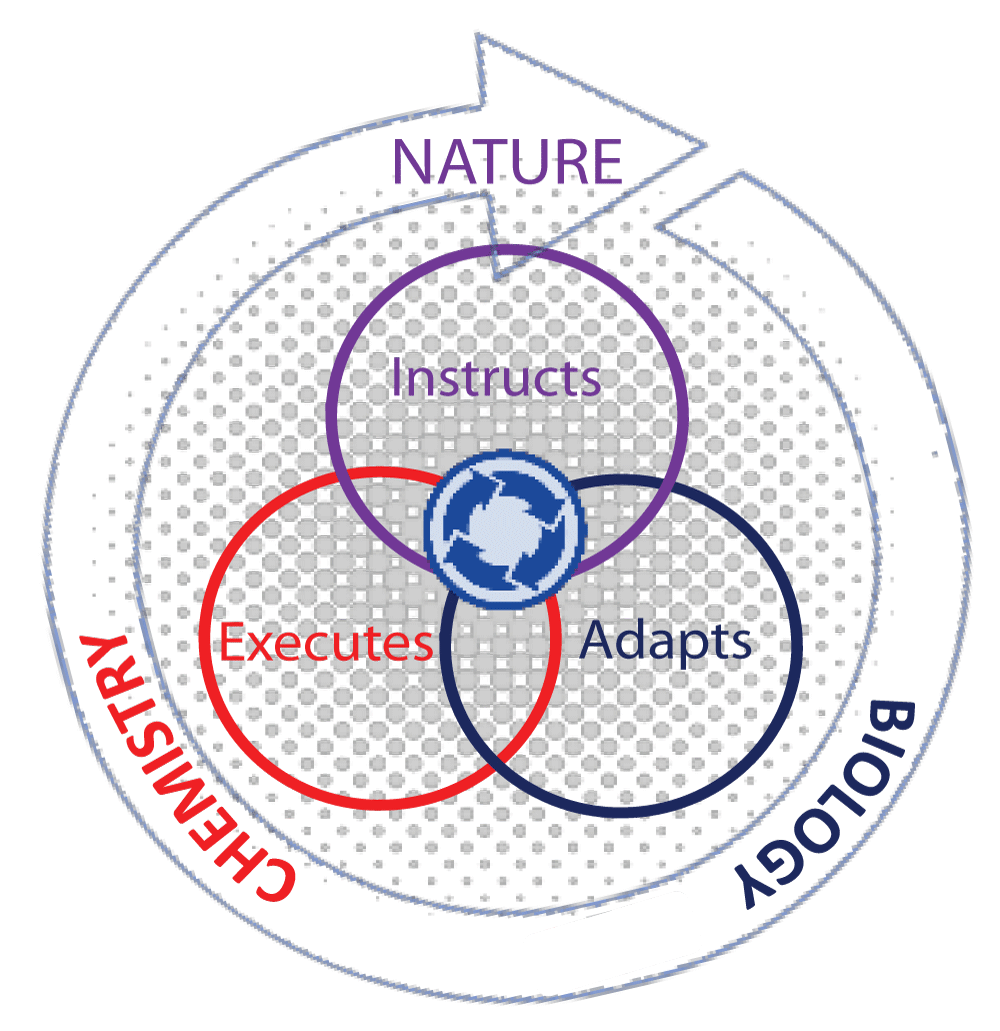 Figure 5: A model of connectivity that allows organisms to r...
Figure 5: A model of connectivity that allows organisms to r...
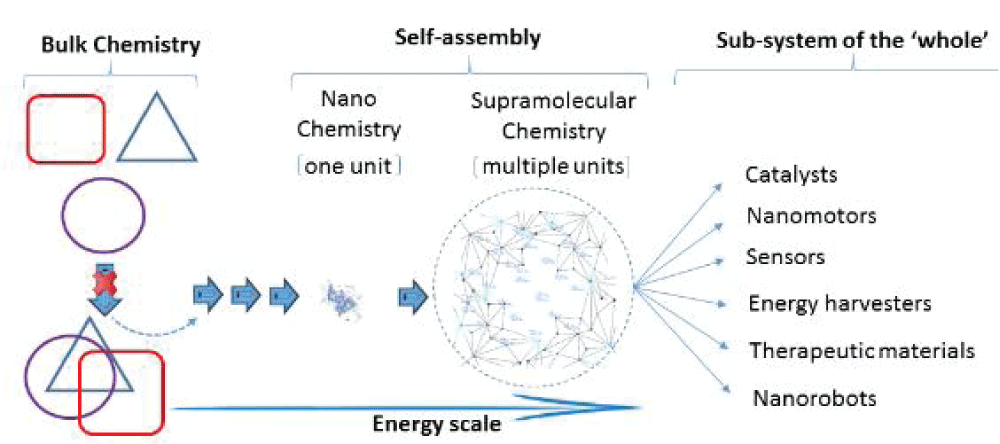 Figure 6: A schematic vision of bulk chemical components and...
Figure 6: A schematic vision of bulk chemical components and...
Olson S. Through teacher outreach programs: a workshop summary to the Chemical Sciences Roundtable. In: The high school chemistry teacher: status and outlook. Washington (DC): National Academy of Sciences; 2009;70. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26411/
Taylor EM, Mitchell R, Drennan CL. Creating an interdisciplinary introductory chemistry course without time-intensive curriculum changes. ACS Chem Biol. 2009 Dec 18;4(12):979-82. doi: 10.1021/cb9002927. PMID: 20017574; PMCID: PMC5920689.
Taniewski M. Chemistry is an interdisciplinary science and is very important for many other disciplines. Chemik. 2010;64:1–5.
Nagle B. Preparing high school students for the interdisciplinary nature of modern biology. CBE Life Sci Educ. 2013 Jun 1;12(2):144-7. doi: 10.1187/cbe.13-03-0047. PMID: 23737621; PMCID: PMC3671640.
Feszterová M. Interdisciplinary approach for the education of pre-service chemistry teachers. AIP Conf Proc. 2019;2152:030005.
Harackiewicz JM, Smith JL, Priniski SJ. Interest Matters: The Importance of Promoting Interest in Education. Policy Insights Behav Brain Sci. 2016 Oct;3(2):220-227. doi: 10.1177/2372732216655542. Epub 2016 Jun 30. PMID: 29520371; PMCID: PMC5839644.
Drinkwater MJ, Matthews KE, Seiler J. How Is Science Being Taught? Measuring Evidence-Based Teaching Practices across Undergraduate Science Departments. CBE Life Sci Educ. 2017 Spring;16(1):ar18. doi: 10.1187/cbe.15-12-0261. PMID: 28232589; PMCID: PMC5332044.
Klein JT. Interdisciplinarity: history, theory, and practice. Detroit (MI): Wayne State University Press; 1990.
Gallagher R, Appenzeller T. Beyond reductionism. Science. 1999;284:79.
Chang H. In: Arabatzis T, Renn J, Simões A, editors. Relocating the history of science: essays in honor of Kostas Gavroglu. Cham (CH): Springer International Publishing; 2015;193–209.
Anastas PT. Beyond reductionist thinking in chemistry for sustainability. Trends Chem. 2019;1:145–8.
Wrigley T. The problem of reductionism in educational theory: complexity, causality, values. Power Educ. 2019;11:145–62.
Morris CW. The unity of science movement and the United States. Synthese. 1938;3:25–9.
Dewey J. Experience and education. New York: The Macmillan Company; 1938.
Bloom BS. Ideas, problems, and methods of inquiry. In: The integration of educational experiences. Yearbook of the National Society for the Study of Education. 1958;57:84–104.
Jenkins EW. From Armstrong to Nuffield. London: John Murray; 1979.
Johnstone AH. The development of chemistry teaching. J Chem Educ. 1993;70:701.
Dunkel HB. Herbartianism comes to America: Part I. Hist Educ Q. 1969;9:202–33.
Walker DF. [Book review]. Am J Educ. 1987;95:495–8.
Meeting WCL. Chemistry in a multidisciplinary, interdisciplinary world. J Chem Educ. 2017;39:43.
Loughran J. Pedagogy: making sense of the complex relationship between teaching and learning. Curriculum Inq. 2013;43:118–41.
Fadillah A, Annisa D, Ad'hiya E, Laksmi N, Dewi C, Sadhu S. Developing students' global awareness through chemical literacy: problems and possibilities. 2018.
McGill TL, Williams LC, Mulford DR, Blakey SB, Harris RJ, Kindt JT, et al. Chemistry unbound: designing a new four-year undergraduate curriculum. J Chem Educ. 2019;96:35–46.
Fowler WC, Ting JM, Meng S, Li L, Tirrell MV. Integrating systems thinking into teaching emerging technologies. J Chem Educ. 2019;96:2805–13.
Pazicni S, Flynn AB. Systems thinking in chemistry education: theoretical challenges and opportunities. J Chem Educ. 2019;96:2752–63.
Talanquer V. Some insights into assessing chemical systems thinking. J Chem Educ. 2019;96:2918–25.
York S, Lavi R, Dori YJ, Orgill M. Applications of systems thinking in STEM education. J Chem Educ. 2019;96:2742–51.
Swiegers G, editor. Bioinspiration and biomimicry in chemistry: reverse-engineering nature. New York: John Wiley & Sons; 2012.
Bodner GM. Constructivism: a theory of knowledge. J Chem Educ. 1986;63:873.
Kamińska K, Szczylik C, Bielecka ZF, Bartnik E, Porta C, Lian F, Czarnecka AM. The role of the cell-cell interactions in cancer progression. J Cell Mol Med. 2015 Feb;19(2):283-96. doi: 10.1111/jcmm.12408. Epub 2015 Jan 19. PMID: 25598217; PMCID: PMC4407603.
Koutsogiannouli E, Papavassiliou AG, Papanikolaou NA. Complexity in cancer biology: is systems biology the answer? Cancer Med. 2013 Apr;2(2):164-77. doi: 10.1002/cam4.62. Epub 2013 Feb 17. PMID: 23634284; PMCID: PMC3639655.
Archer TC, Fertig EJ, Gosline SJ, Hafner M, Hughes SK, Joughin BA, Meyer AS, Piccolo SR, Shajahan-Haq AN. Systems Approaches to Cancer Biology. Cancer Res. 2016 Dec 1;76(23):6774-6777. doi: 10.1158/0008-5472.CAN-16-1580. Epub 2016 Nov 18. PMID: 27864348; PMCID: PMC5135591.
Linthorst JA. The image of chemistry and curriculum changes. Educ Quim. 2012;23:240–2.
Sarantopoulos P, Tsaparlis G. Analogies in chemistry teaching as a means of attainment of cognitive and affective objectives: a longitudinal study in a naturalistic setting using analogies with a strong social content. Chem Educ Res Pract. 2004;5:33–50.
Georgiou SM, Georgiou J. Precision agriculture: challenges in sensors and electronics for real-time soil and plant monitoring. 2018;1–4.
Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven challenges for nanomedicine. Nat Nanotechnol. 2008 May;3(5):242-4. doi: 10.1038/nnano.2008.114. PMID: 18654511.
Halappanavar S, Vogel U, Wallin H, Yauk CL. Promise and peril in nanomedicine: the challenges and needs for integrated systems biology approaches to define health risk. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018 Jan;10(1):e1465. doi: 10.1002/wnan.1465. Epub 2017 Mar 15. PMID: 28294555; PMCID: PMC5763403.
Hua S, Wu SY. Editorial: Advances and Challenges in Nanomedicine. Front Pharmacol. 2018 Nov 29;9:1397. doi: 10.3389/fphar.2018.01397. PMID: 30555328; PMCID: PMC6281879.
Grafton RQ, McLindin M, Hussey K, Wyrwoll P, Wichelns D, Ringler C, et al. Responding to global challenges in food, energy, environment and water: risks and options assessment for decision-making. Asia Pac Policy Stud. 2016;3:275–99.
Cooper MM, Stowe RL. Chemistry Education Research-From Personal Empiricism to Evidence, Theory, and Informed Practice. Chem Rev. 2018 Jun 27;118(12):6053-6087. doi: 10.1021/acs.chemrev.8b00020. Epub 2018 Jun 12. PMID: 29893111.
Windschitl M. Framing constructivism in practice as the negotiation of dilemmas: an analysis of the conceptual, pedagogical, cultural, and political challenges facing teachers. Rev Educ Res. 2002;72:131–75.
Taber KS. The challenge of teaching and learning chemical concepts. Cambridge (UK): The Royal Society of Chemistry; 2019.
Sebastian V, Gimenez M. Teaching nanoscience and thinking nano at the macroscale: nanocapsules of wisdom. Procedia Soc Behav Sci. 2016;228:489–95.
Bradforth SE, Miller ER, Dichtel WR, Leibovich AK, Feig AL, Martin JD, Bjorkman KS, Schultz ZD, Smith TL. University learning: Improve undergraduate science education. Nature. 2015 Jul 16;523(7560):282-4. doi: 10.1038/523282a. PMID: 26178951.
Harris HH. Chemical misconceptions—prevention, diagnosis and cure; Volume I: Theoretical background; Volume II: Classroom resources (Taber, Keith). J Chem Educ. 2003;80:491.
Castleman AW Jr, Jena P. Clusters: a bridge between disciplines. Proc Natl Acad Sci U S A. 2006 Jul 11;103(28):10552-3. doi: 10.1073/pnas.0601783103. PMID: 16835304; PMCID: PMC1502271.
Sonkaria S, Lee J, Khare V. The potential of multi-functional nanostructured components for soft robotic medical applications. In: Proceedings of the 19th International Conference on Control, Automation and Systems (ICCAS); 2019.
Sonkaria S, Fuentes G, Verma C, Narang R, Khare V, Fischer A, Faivre D. Insight into the assembly properties and functional organisation of the magnetotactic bacterial actin-like homolog, MamK. PLoS One. 2012;7(5):e34189. doi: 10.1371/journal.pone.0034189. Epub 2012 May 7. PMID: 22586444; PMCID: PMC3346761.
Lin W, Paterson GA, Zhu Q, Wang Y, Kopylova E, Li Y, Knight R, Bazylinski DA, Zhu R, Kirschvink JL, Pan Y. Origin of microbial biomineralization and magnetotaxis during the Archean. Proc Natl Acad Sci U S A. 2017 Feb 28;114(9):2171-2176. doi: 10.1073/pnas.1614654114. Epub 2017 Feb 13. PMID: 28193877; PMCID: PMC5338559.
Monat JP. Explaining natural patterns using systems thinking. Am J Syst Sci. 2018;6:1–15.
Khare V, Sonkaria S. Nanobiomimicry at quantum scales: synthetic imperfections to imitating low-dimensional biomimetic polymer confinement of TiO2 at self-assembled heterogeneous interfaces. Amsterdam: Elsevier; 2018.
Whitesides GM, Boncheva M. Beyond molecules: self-assembly of mesoscopic and macroscopic components. Proc Natl Acad Sci U S A. 2002 Apr 16;99(8):4769-74. doi: 10.1073/pnas.082065899. PMID: 11959929; PMCID: PMC122665.
Kohn KP, Underwood SM, Cooper MM. Energy Connections and Misconnections across Chemistry and Biology. CBE Life Sci Educ. 2018 Spring;17(1):ar3. doi: 10.1187/cbe.17-08-0169. PMID: 29351907; PMCID: PMC6007765.