Abstract
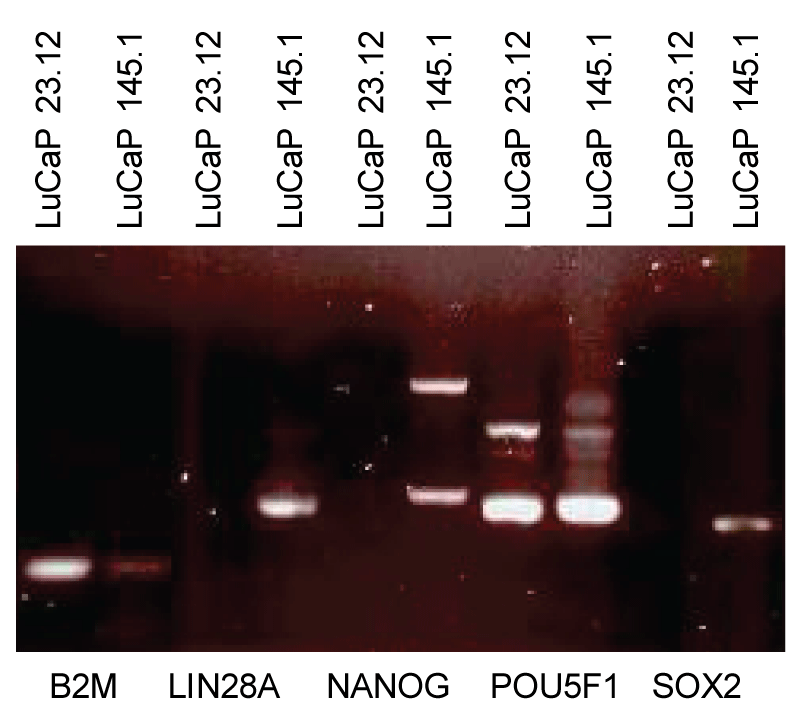
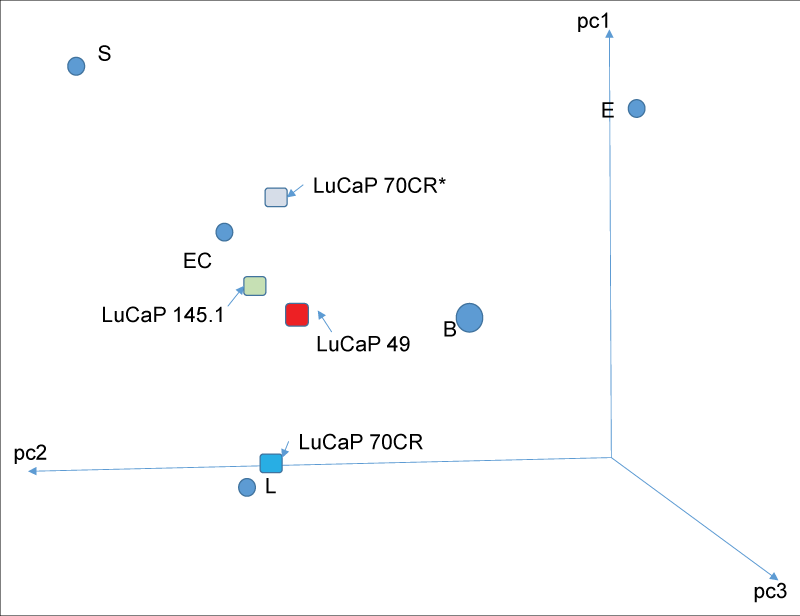
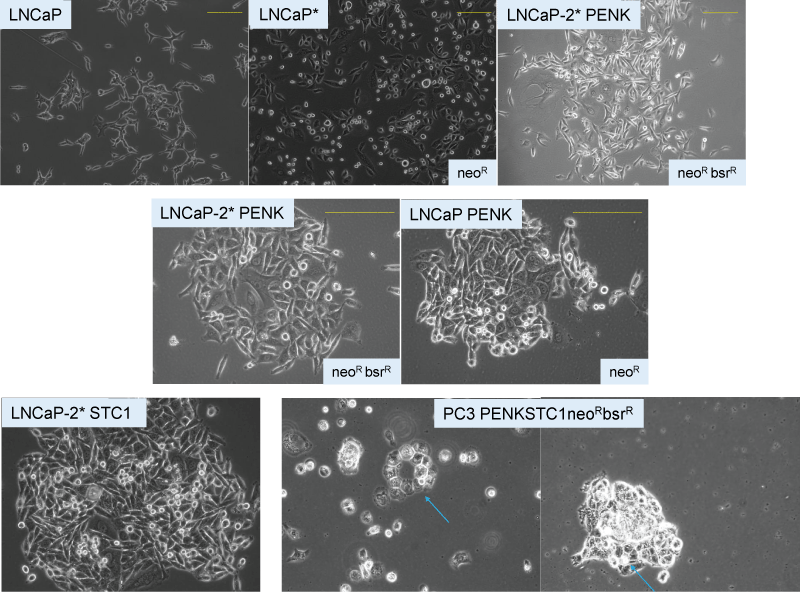
The cancer stem cell (CSC) hypothesis proposes that rare tumor-initiating cells with stem-like properties drive cancer progression and metastasis. Through comprehensive analysis of prostate cancer patient-derived xenografts (PDX), we demonstrate that all cancer cell types—not exclusively stem-like cells—can initiate tumors in mice, contradicting a core CSC tenet. CD44, commonly used to identify CSCs, proved inconsistent in prostate cancer. However, stem-like cancer cells do exist, characterized by expression of stem cell transcription factors (LIN28A, NANOG, POU5F1, SOX2) and low β-2 microglobulin (scTF⁺B2Mlo). We show that differentiated adenocarcinomas can be experimentally reprogrammed to stem-like small cell carcinomas through scTF expression, while stromal signaling molecules like proenkephalin (PENK) induce differentiation. These findings reveal that prostate cancer progression involves dynamic dedifferentiation due to loss of stromal signaling rather than clonal expansion of rare CSCs. Cancer cells exhibit remarkable plasticity, undergoing reversible differentiation-dedifferentiation cycles independent of mutation burden, suggesting differentiation therapy as a promising treatment strategy.